HOME
RESEARCH
PUBLICATIONS
PEOPLE
CONTACT INFO
ProteoSync
Overview:
The research focus of the Sicheri lab is to understand how Eukaryotic signaling proteins function by visualizing and characterizing snapshots of these proteins in action. We use a combination of X-ray crystallography, protein NMR spectroscopy, and cryo-EM approaches as our primary tools for structure analysis.
We study the structural biology of two broad classes of signal transduction molecules, namely the eukaryotic protein kinases and proteins in the ubiquitin proteasome system (UPS). We strive to understand the structural basis by which these signaling proteins act to regulate normal cellular behaviour and mechanisms of dysregulation that cause human disease.
Our ultimate goal is to translate our discoveries to the benefit of human health. This is done by 1) collaborating with medicinal chemists to understand drug mechanism of action, 2) investigating the "druggability" of specific protein targets, 3) generating tool compounds to probe biology and validate drug targets, and lastly 4) generating structure platforms to guide the development of more potent therapeutics.
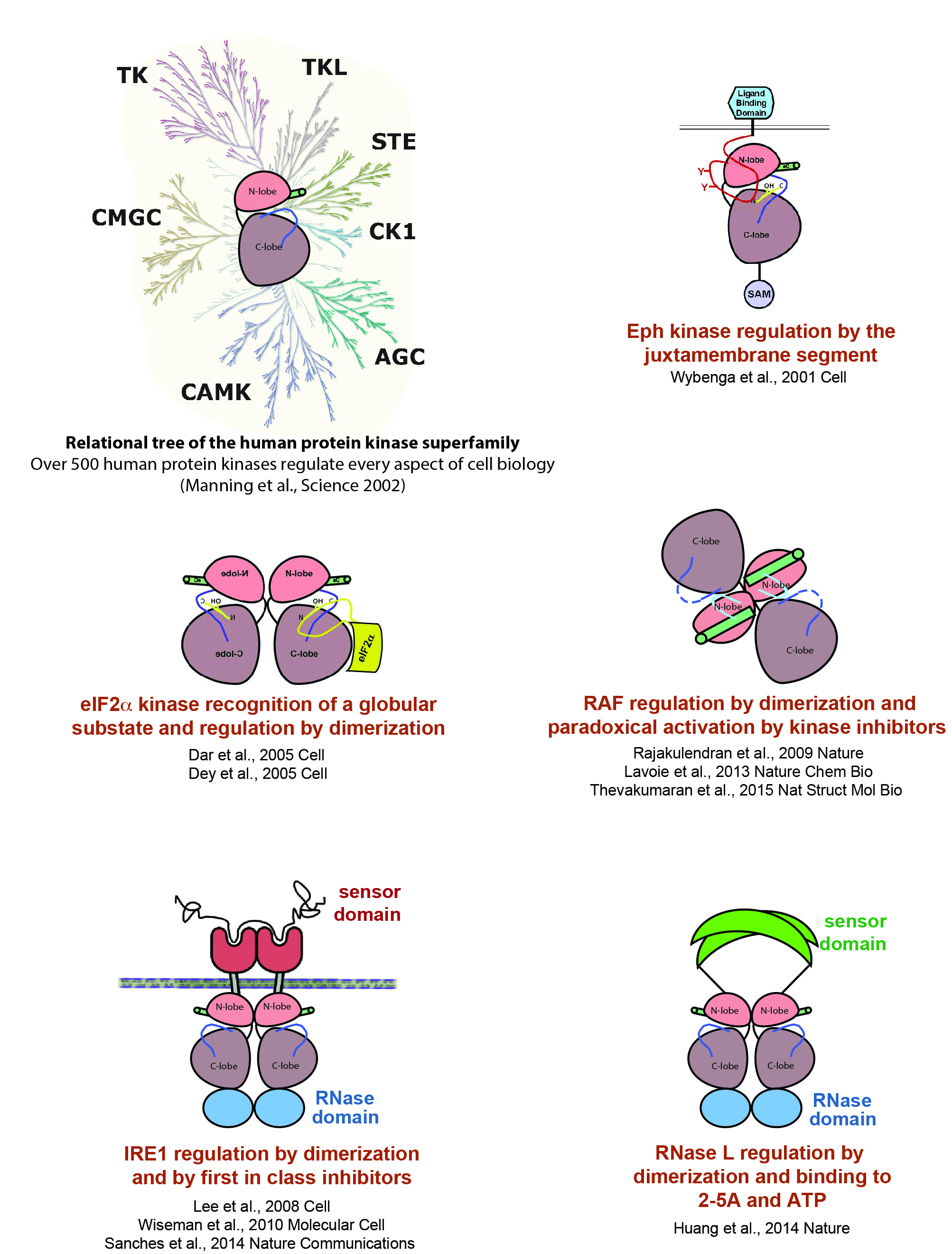
Protein Kinase Research:
A great plasticity of structure and domain architecture within this group of proteins allows for the diversification of catalytic switching (ie on/off) and substrate recognition mechanisms. Our research objectives include addressing how disease-associated protein kinases are regulated by intra and intermolecular mechanisms. Examples of our work in this area are shown below.
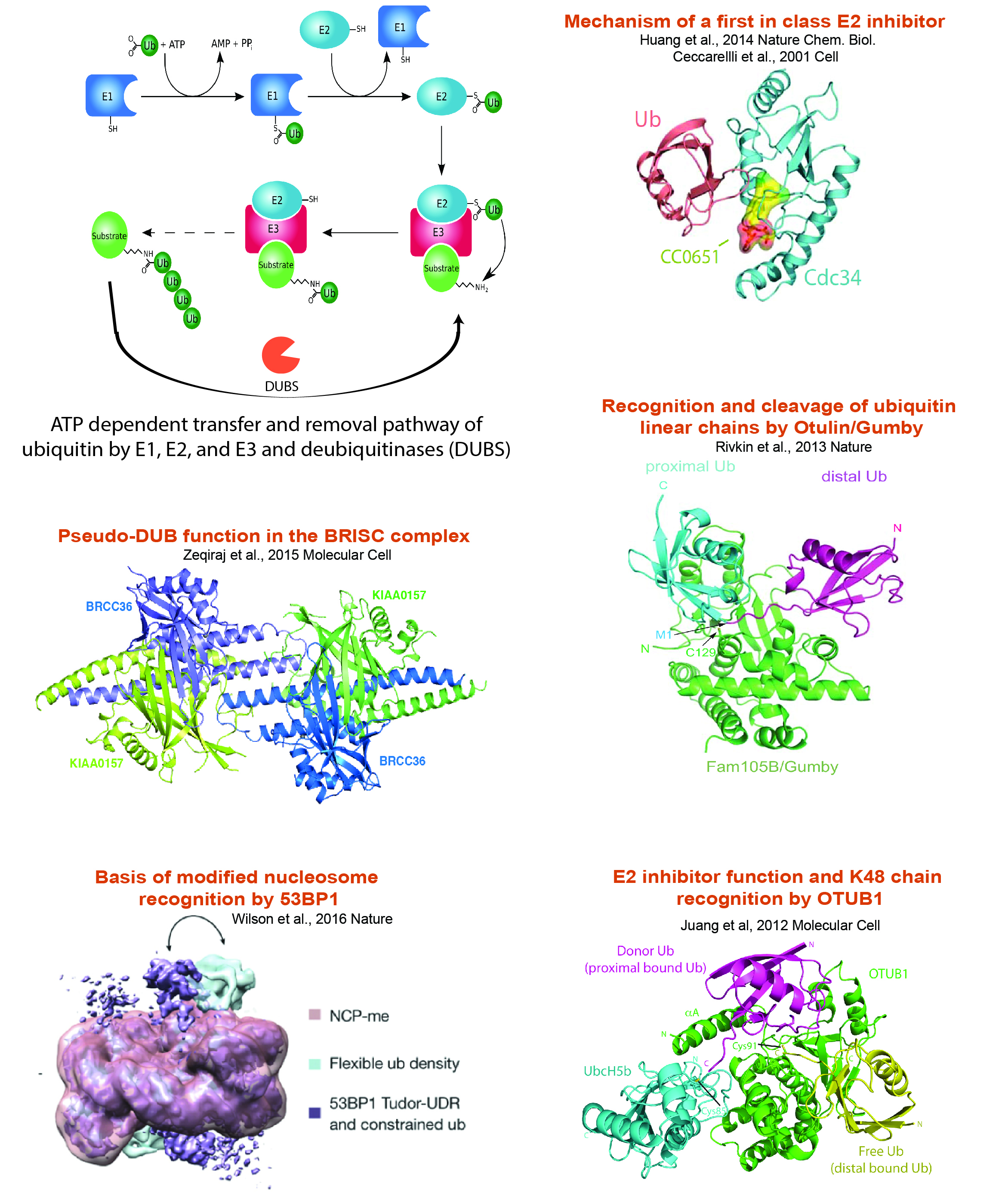
Ubiquitin Proteasome System Research:
Protein ubiquitination influences the spatial and temporal control of protein stability, activity or localization. The conserved E1->E2->E3 enzyme cascade, comprised of over 700 protein components, activates and transfers ubiquitin through step-wise thioester linkages that end in covalent conjugation of ubiquitin to free amino groups on target protein substrates. Our research objectives include addressing how ubiquitin addition and removal occurs, the effect of ubiquitination on target protein function, and how the networks of supporting protein interactions and catalytic activities are regulated. Examples of our work in this area are shown below.