SAPK/Jun kinase assays1. For cells in suspension, grow in DMEM with 10% FCS and then the day before you do the experiment spin the cells down (1 K for 5 min) and obtain a concentration of cells at 2 - 10 x 106 cells per sample, resuspended in DMEM, 2 % FCS. This starves the cells or decreases the background. Then incubate at 37oC, overnight or 16 hrs. For adherent cells, simply change the medium to 2% FCS the night before the expt. 2. The next day, aliquot the 2 - 10 million cells per sample. Cells in 1 ml of the 2% FCS, DMEM, transfer to an Eppendorf tube and stimulate with agonists of choice, for the required time and temperature. 3. Spin for 5 - 10 min @ 1000x g at room temperature. Remove the supernate and add 500 µl to 1 ml of the Hypotonic Lysis solution or Gentle Soft Buffer. Both forms of lysis work equally as well for most cells but I usually use Gentle Soft Buffer. Put on ice for 10 - 20 min., vortexing every few min for lysis of cells and homogenize as noted below. For monolayer cells, which are usually grown in 100 mm plate, 80 - 90% confluent, add 1 ml of lysis buffer and let sit on ice for 20 min in plate. Then scrape the cells off of the plate and homogenize. We use a tissue homogenizer but the needle/syringe method can also work. 4. Spin @ 4 oC for 10 min. @ 13K. Remove sup and put into a new Eppendorf, put on ice or add directly to the reaction of choice (see below). This lysate can be snap frozen and then stored at -70 oC for use in the future but is best made fresh each time. 5. I usually do a Bio Rad protein assay to find the total protein involved in each sample. Add 10 ul of the 1 ml lysate to 1 ml of the diluted dye. Assays: there are two One depends on binding of the kinases to GST-Jun (due to very high affinity) and the other is an IP kinase reaction. Substrate/Antibody, Lysate Interaction: 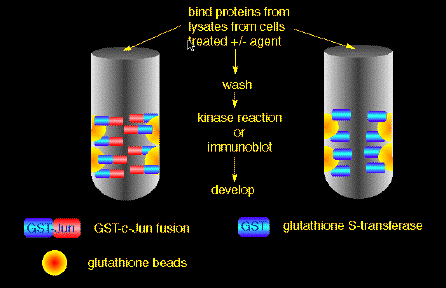 1. For GST c-Jun substrate interaction the quantity of protein on the beads is variable with the batch made (make fresh at least once a week). Generally its between 3 - 20 ul of a 50 % slurry of beads/PBST. Aim for 10-20 µg/assay (determined by running an aliquot on a gel and Coomassie staining). Preparation of GST-Jun beads is exactly as described in the original papers. We tend to use BL21(DE3) pLysS bacteria simply due to their ease of lysis. Other bacteria (e.g. XL-1Blue) are fine. Before the assay, prewash the Jun beads in PBST. 2. If you are only doing a substrate (eg. c-Jun kinase assay) interaction then add the 1 ml of lysate to the aliquot of beads/substrate (beads/GST-Jun). For an immunoprecipitation (IP) interaction I usually add 2 ul of SAPK antibody to the 1 ml lysate. 3. Place all of the samples (IP and substrate assay) on the rotary shaker for 1 hr. at 4 oC, rotating at a speed that will allow the beads/lysate to successfully move back and forth in the tube when turning. If IP is being done add 40 µl 1:1 slurry of Protein A Sepharose beads for an additional 30 minutes. 4. After incubation spin for 1 min in the cold room and wash thoroughly 3-4 x with PBST. 5. Remove all of the PBST and add equal volume of the kinase buffer to the beads, if doing a c-Jun or a substrate kinase assay. For the IP kinase assay add appropriate quantity of soluble c-Jun* or substrate (such that both proteins, substrate/beads or substrate/soluble are equal in quantity when ran on a gel) to the protein A beads/antibody + kinase buffer and leave at 30oC for 20 - 30 min. 6. Stop reaction by addition of 40 µl 2x sample buffer and boil for 5 min. 7. Run 1/2 of the mixture on a polyacrylamide gel (12.5%). Stain, destain, expose. *Prepared by eluting GST-Jun from beads with 10 mM reduced glutathione, pH 7.5 followed by dialysis against Tris-Cl pH 7.5 + 1 mM DTT + 10% glycerol. BUFFERS (all concentrations are final) PBST - Store in fridge KINASE BUFFER - Store in freezer GENTLE SOFT BUFFER - Store in fridge |